RESEARCH
Our lab focuses on neural regenerative medicine. We take an interdisciplinary approach to exploring and enhancing the regenerative potential of the central nervous system. This includes harnessing the potential of neural stem cells and their progeny as well as using gene therapy approaches to replace lost cells.
To harness the potential of neural stem cells, it is critical to understand their fundamental biology. With this understanding we can consider both exogenous cell transplantation paradigms as well as endogenous strategies to activate the resident precursors pools in pre-clinical animal models of injury/disease. In considering the potential success and clinical translation of our neural repair strategies, we use cellular, structural and functional outcomes as measures of success.
Here are some of the “big picture” projects we are pursuing!
A) Stem Cell Biology:
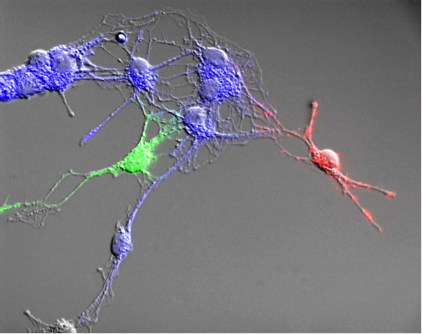
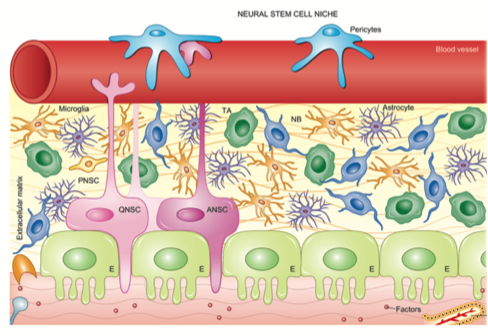
We are interested in the mechanisms that regulate neural stem and progenitor cell behaviour. We have recently found a novel stem cell in the developing nervous system that persists into adulthood along the entire neuraxis, essentially redefining the neural stem cell lineage in the CNS. We use in vitro and in vivo assays, transgenic mouse models and novel tools to explore stem cell behaviour and the niche cells that regulate stem cell kinetics, migration and differentiation. We want to apply this knowledge to design novel regenerative medicine strategies.
B) Electric field application to activate neural stem cells:
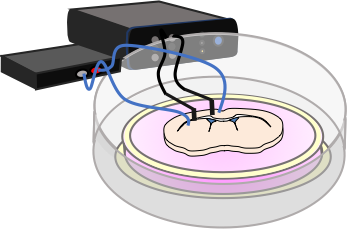
We have determined that neural stem cells and their progeny are electrosenstive cells and their behaviour can be modified by the application of clinically relevant electric fields to promote migration, proliferation and differentiation of cells. We have shown this using culture assays and in the rodent brain using implanted electrodes. We have a multidisciplinary, innovative team which is developing novel, multimodal electroceuticals to stimulate neural precursor cells and repair the injured central nervous system.
C) Stem Cells to Treat the Injured Nervous System:
The potential for stem cells to treat a variety of currently untreatable human diseases has received worldwide attention. In particular, research in animal models and some early clinical trials have stem cells could potentially be used to treat patients suffering from disorders of the nervous system, such as stroke and spinal cord injury, conditions which are devastating to the patients, their families, and society as a whole. Transplantation studies using neural stem and progenitor populations are one strategy to promote tissue repair and we are actively pursuing these paradigms with novel cell sources combined with bioengineering strategies.
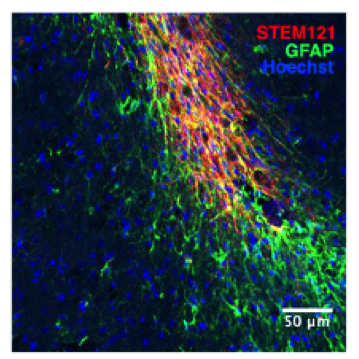
C-1) Stroke:
Stroke can happen at any age. While most prominent in the aged population, it can occur perinatally and into adulthood. We are using a number of stroke models and taking two stem cell based approaches to promote repair of the stroke injured brain. First, the use of factors to activate stem cells that are already resident in the mammalian brain to ask whether this can promote “self-repair” mechanisms. Behavioural assays for sensory-motor function as well as cognition are employed- arguably the most clinically relevant outcome measurements. In adult models of stroke we are transplanting clinically relevant human derived neural precursor cells into the injured brains and asking important questions about the mechanisms by which the transplanted cells support recovery. We want to know if cell integration into the host tissue is required for functional recovery or whether the transplanted cells mediate their benefit by releasing factors that promote host plasticity. These are fundamental questions that will help to focus future stem cells based therapies to treat stroke.
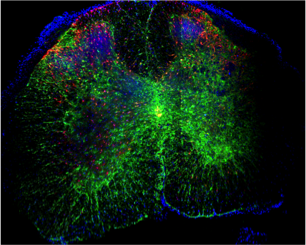
C-2) Spinal Cord Injury:
Spinal cord injury (SCI) is a devastating event with major social and economic implications. We are using a combination of strategies including tissue engineering and endogenous stem cell activation in an attempt to achieve functionally significant repair and regeneration in rodent models of SCI. In collaboration with neurosurgeons and bioengineers we are combining our efforts to replace lost cells; create devices that promote neural stem cell migration and provide an local environment that promotes repair and recovery following neurotrauma.
D) Cellular Reprogramming to promote neurorepair:
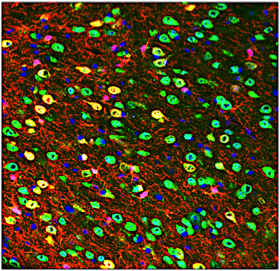
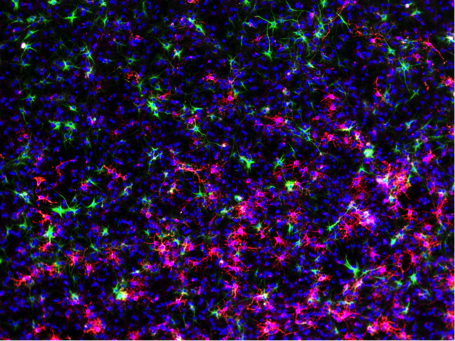
Damage to the central nervous system, including the brain or spinal cord, can be triggered by trauma or neurodegenerative disease. The resulting death of neurons leads to motor and cognitive impairments with serious repercussions which are largely irreversible. We have developed novel gene therapy approaches to convert glial cells in the brain (that are not lost after injury) into new neurons to replace those that have died. Our collaborative team uses viral delivery and novel vectors to accomplish the cellular reprogramming. The promise of this therapy is to treat millions of people who suffer from devastating neurotrauma and progressive disease and improve quality of life.